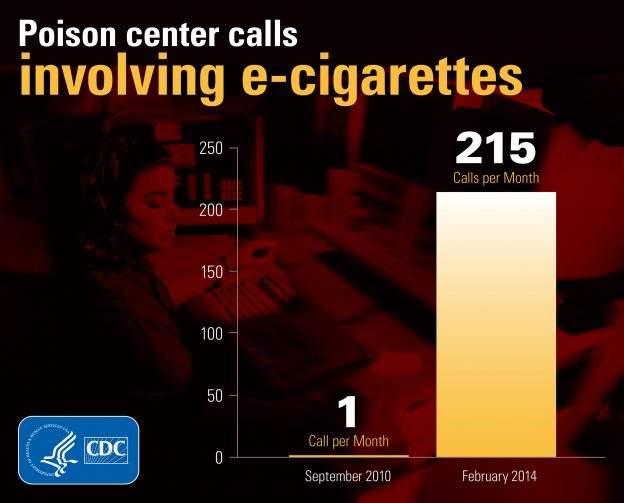
“The Effects and Hazards of E-cigarettes: How and Why Public Health Departments Should Inform Patients Now” Because there is still the effect of “the jury is out” about the very specific long-term effects of e-cigarettes, it’s important that public health advocates and doctors communicate with their patients about counteracting the marketing that they’ve seen, that […]
Read More
